- Because Atoms Are Electrically Neutral The Number Of Protons And
- Atoms Are Neutral Because The Number Of Regents
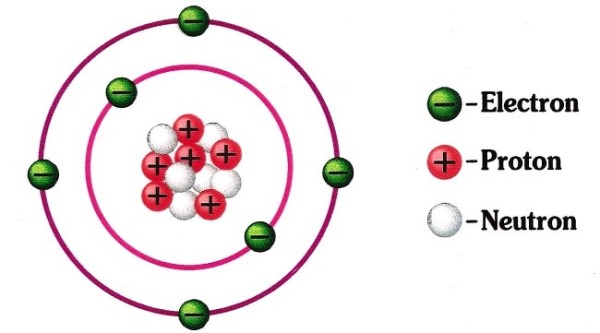
Atoms, as a whole, are considered neutral because the atom of any element has equal number of protons (positively charged particles) and electrons (negatively charged particles), canceling out and making the atom neutral as whole. Also, the neutrons are called so because they are neutral, that is they don’t hold any charge at all.

. Atoms of an element that contain the same number of protons but a different number of neutrons are called isotopes of that element. (3.1m) Hints for Facilitation Exercises: A Limiting response to a student question might be – Atoms are neutral because the number of protons equals the number of electrons. Atoms are neutral because they have same number of positively charged particles, protons, and negatively charged particles, electrons. The amount of charge in a single proton is equal to the amount of charge possessed by a single electron. Atoms also have another particle type in the nucleus called neutrons that have no charge. Contactygopro for mac.
An atom is considered the most basic unit of matter, and it is considered neutral or neutrally-charged simply because it contains the same numbers of protons and electrons. Â Protons and electrons have opposite charges, but they basically cancel out each other making the charge neutral.
There are actually three particles that make up an atom and these are; neutrons, protons, and electrons. Â The outer part of an atom is formed by multiple electrons that have negative charges. Â The inner portion, called the nucleus, is meanwhile composed of the other two particles called protons and neutrons. Neutrons are those that have a neutral charge as the name suggests. Â Protons, meanwhile, contain a positive electrical charge. Â For a typical atom, the number of protons at the nucleus always equals the number of electrons that surround them. Best free dvd video burning software macsitevivid. So even if the protons and electrons contain opposite electrical charges, they wouldn’t be able to cause a reaction because their charges will even out with each other. Â Because of this configuration in terms of the proton and electron charge, the atom will contain a neutral charge.

Under normal conditions, the placement of atomic particles stays where it is, and that is referring to the protons and neutrons lying at the center, or nucleus, while the electrons are formed on the outside parts. Â This basic atomic structure is said to be maintained by an electromagnetic force. Â If there is no such force, then the particles will not be bound together. Â In the same way, all atoms that are electromagnetically bound together form a bigger substance called a molecule. Â The charge for a molecule depends on whether the atoms combined are positively or negatively charged. Â Atoms that are positively charged or negatively charged become an ion. Â But in standard conditions, the charge of an atom remains neutral because of the equal number of positive protons and negative electrons.
Because Atoms Are Electrically Neutral The Number Of Protons And
Author: Hari M
Atoms Are Neutral Because The Number Of Regents
