
How did Rutherford figure out the structure of the atom without being able to see it? Simulate the famous experiment in which he disproved the Plum Pudding model of the atom by observing alpha particles bouncing off atoms and determining that they must have a small core. Rutherford’s basic model by proposing that electrons had set energy levels (Fig. This is the model of the atom most commonly portrayed in textbooks: a nucleus orbited by electrons at different levels. It helped solve the problem of the collapsing atom and earned Bohr a Nobel Prize. Just as Bohr built on Rutherford’s model, many other. The Rutherford model was devised by the New Zealand-born physicist Ernest Rutherford to describe an atom.Rutherford directed the Geiger–Marsden experiment in 1909, which suggested, upon Rutherford's 1911 analysis, that J. Thomson's plum pudding model of the atom was incorrect.
Our editors will review what you’ve submitted and determine whether to revise the article.
Join Britannica's Publishing Partner Program and our community of experts to gain a global audience for your work!Ernest Rutherford, in full Ernest, Baron Rutherford of Nelson, (born August 30, 1871, Spring Grove, New Zealand—died October 19, 1937, Cambridge, Cambridgeshire, England), New Zealand-born British physicist considered the greatest experimentalist since Michael Faraday (1791–1867). Rutherford was the central figure in the study of radioactivity, and with his concept of the nuclear atom he led the exploration of nuclear physics. He won the Nobel Prize for Chemistry in 1908, was president of the Royal Society (1925–30) and the British Association for the Advancement of Science (1923), was conferred the Order of Merit in 1925, and was raised to the peerage as Lord Rutherford of Nelson in 1931.
What did Ernest Rutherford discover about the atom?
Ernest Rutherford found that the atom is mostly empty space, with nearly all of its mass concentrated in a tiny central nucleus. The nucleus is positively charged and surrounded at a great distance by the negatively charged electrons.
What is Ernest Rutherford most famous for?
Ernest Rutherford is known for his pioneering studies of radioactivity and the atom. He discovered that there are two types of radiation, alpha and beta particles, coming from uranium. He found that the atom consists mostly of empty space, with its mass concentrated in a central positively charged nucleus.
What is Ernest Rutherford’s most famous experiment?
Ernest Rutherford’s most famous experiment is the gold foil experiment. A beam of alpha particles was aimed at a piece of gold foil. Most alpha particles passed through the foil, but a few were scattered backward. This showed that most of the atom is empty space surrounding a tiny nucleus.
Early life and education
Rutherford’s father, James Rutherford, moved from Scotland to New Zealand as a child in the mid-19th century and farmed in that agrarian society, which had only recently been settled by Europeans. Rutherford’s mother, Martha Thompson, came from England, also as a youngster, and worked as a schoolteacher before marrying and raising a dozen children, of whom Ernest was the fourth child and second son.
Ernest Rutherford attended the free state schools through 1886, when he won a scholarship to attend Nelson Collegiate School, a private secondary school. He excelled in nearly every subject, but especially in mathematics and science.
Another scholarship took Rutherford in 1890 to Canterbury College in Christchurch, one of the four campuses of the University of New Zealand. It was a small school, with a faculty of eight and fewer than 300 students. Rutherford was fortunate to have excellent professors, who ignited in him a fascination for scientific investigation tempered with the need for solid proof.
On conclusion of the school’s three-year course, Rutherford received a bachelor of arts (B.A.) degree and won a scholarship for a postgraduate year of study at Canterbury. He completed this at the end of 1893, earning a master of arts (M.A.) degree with first-class honours in physical science, mathematics, and mathematical physics. He was encouraged to remain yet another year in Christchurch to conduct independent research. Rutherford’s investigation of the ability of a high-frequency electrical discharge, such as that from a capacitor, to magnetize iron earned him a bachelor of science (B.S.) degree at the end of 1894. During this period he fell in love with Mary Newton, the daughter of the woman in whose house he boarded. They married in 1900.
In 1895 Rutherford won a scholarship that had been created with profits from the famous Great Exhibition of 1851 in London. He chose to continue his study at the Cavendish Laboratory of the University of Cambridge, which J.J. Thomson, Europe’s leading expert on electromagnetic radiation, had taken over in 1884.
University of Cambridge
In recognition of the increasing importance of science, the University of Cambridge had recently changed its rules to allow graduates of other institutions to earn a Cambridge degree after two years of study and completion of an acceptable research project. Rutherford became the school’s first research student. Besides showing that an oscillatory discharge would magnetize iron, which happened already to be known, Rutherford determined that a magnetized needle lost some of its magnetization in a magnetic field produced by an alternating current. This made the needle a detector of electromagnetic waves, a phenomenon that had only recently been discovered. In 1864 the Scottish physicist James Clerk Maxwell had predicted the existence of such waves, and between 1885 and 1889 the German physicist Heinrich Hertz had detected them in experiments in his laboratory. Rutherford’s apparatus for detecting electromagnetic waves, or radio waves, was simpler and had commercial potential. He spent the next year in the Cavendish Laboratory increasing the range and sensitivity of his device, which could receive signals from half a mile away. However, Rutherford lacked the intercontinental vision and entrepreneurial skills of the Italian inventor Guglielmo Marconi, who invented the wirelesstelegraph in 1896.
X-rays were discovered in Germany by physicist Wilhelm Conrad Röntgen only a few months after Rutherford arrived at the Cavendish. For their ability to take silhouette photographs of the bones in a living hand, X-rays were fascinating to scientists and laypeople alike. In particular, scientists wished to learn their properties and what they were. Rutherford could not decline the honour of Thomson’s invitation to collaborate on an investigation of the way in which X-rays changed the conductivity of gases. This yielded a classic paper on ionization—the breaking of atoms or molecules into positive and negative parts (ions)—and the charged particles’ attraction to electrodes of the opposite polarity.
Thomson then studied the charge-to-mass ratio of the most common ion, which later was called the electron, while Rutherford pursued other radiations that produced ions. Rutherford first looked at ultraviolet radiation and then at radiation emitted by uranium. (Uranium radiation was first detected in 1896 by the French physicist Henri Becquerel.) Placement of uranium near thin foils revealed to Rutherford that the radiation was more complex than previously thought: one type was easily absorbed or blocked by a very thin foil, but another type often penetrated the same thin foils. He named these radiation types alpha and beta, respectively, for simplicity. (It was later determined that the alpha particle is the same as the nucleus of an ordinary helium atom—consisting of two protons and two neutrons—and the beta particle is the same as an electron or its positive version, a positron.) For the next several years these radiations were of primary interest; later the radioactiveelements, or radioelements, which were emitting radiation, enjoyed most of the scientific attention.
- born
- August 30, 1871
Spring Grove, New Zealand
- died
- October 19, 1937 (aged 66)
Cambridge, England
- subjects of study
- Copley Medal (1922)
- Nobel Prize (1908)
Main Difference – Rutherford vs Bohr Model
Rutherford model and Bohr model are models that explain the structure of an atom. Rutherford model was proposed by Ernest Rutherford in 1911. Bohr model was proposed by Niels Bohr in 1915. Bohr model is considered as a modification of Rutherford model. The main difference between Rutherford and Bohr model is that Rutherford model does not explain the energy levels in an atom whereas Bohr model explains the energy levels in an atom.
Key Areas Covered
1. What is Rutherford Model
– Definition, Explanation of the Model
2. What is Bohr Model
– Definition, Explanation of the Model
3. What is the Difference Between Rutherford and Bohr Model
– Comparison of Key Differences
Key Terms: Alpha Particles, Atom, Bohr Model, Electron, Line Spectra, Nucleus, Orbitals, Rutherford Model
What is Rutherford Model
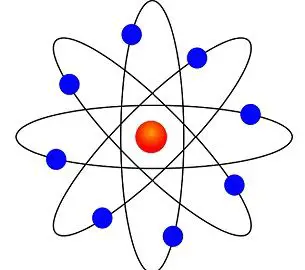
Rutherford model of atom describes that an atom is composed of a central core and nearly all mass of that atom is concentrated and light weight particles move around this central core. It also states that the central core is positively charged and constituents that move around the central core are negatively charged.
Figure 1: Structure of Atom as Suggested by Rutherford
This model was experimentally observed by Ernest Rutherford via the famous “Rutherford gold foil experiment”. In this experiment, alpha particles were bombarded through a gold foil; they were expected to go straight through the gold foil. But instead of straight penetration, alpha particles turned into different directions.
In order to explain this model, Rutherford suggested the followings.
- An atom is composed of a central core which has a positive charge.
- Negatively charged constituents are located around this central core.
- Positive and negative charges balance with each other.
However, this Rutherford model of atom was also rejected because it couldn’t explain why electrons and the positive charges in the nucleus are not attracted to each other.
What is Bohr Model
Bohr model is a modification of the Rutherford model. This model was proposed based on the line spectra of the hydrogen atom. This model proposed that electrons are always traveling in specific shells or orbits around the nucleus. Bohr model also indicated that these shells have different energies and are spherical in shape.
Furthermore, Bohr model explained that electrons in one orbital can move to different orbital by either absorbing energy or releasing energy.
Figure 2: Atomic Structure according to Bohr Model
The line spectra of hydrogen atom had many discrete lines. In order to explain this spectrum, Bohr suggested the followings.
- Electrons move around the nucleus in certain shells or
- These shells have discrete energy levels.
- The energy of an orbit is related to the size of the orbit. Smallest orbit has the lowest energy.
- Electrons can move from one energy level to another.
Although this model perfectly fits the atomic structure of hydrogen atom, there were certain limitations when applying this model to other elements. One such limitation is the inability to explain the Zeeman effect and Stark effect observed in line spectra.
Difference Between Rutherford and Bohr Model
Definition
Rutherford Model: Rutherford model states that an atom is composed of a central core where nearly the whole mass of that atom is concentrated, and light weight particles move around this central core.
Bohr Model: Bohr model explains that the electrons always travel in specific shells or orbits which are located around the nucleus and these shells have discrete energy levels.
Observation
Rutherford Model: Rutherford model was developed based on observations of gold foil experiment.
Bohr Model: Bohr model was developed based on observations of line spectra of the hydrogen atom.
Energy Levels
Rutherford Model:Rutherford model does not describe the presence of discrete energy levels.
Ernest Rutherford Atom
Bohr Model:Bohr model describes the presence of discrete energy levels.
Size of Orbitals
Rutherford Model: Rutherford model does not explain the relationship between orbital size and the energy of the orbital.
Bohr Model: Bohr model explains the relationship between orbital size and the energy of the orbital; smallest orbital has the lowest energy.
Conclusion
Both Rutherford model and Bohr model explain the same concept of atomic structure with slight variations. The main difference between Rutherford model and Bohr model is that Rutherford model does not explain the energy levels in an atom whereas Bohr model explains the energy levels in an atom.
References:
Rutherford Atom Experiment
1. “Rutherford atomic model.” Encyclopædia Britannica, Encyclopædia Britannica, inc., 10 Aug. 2017, Available here.
2. Helmenstine, Anne Marie. “What is the Bohr Model of the Atom?” ThoughtCo, Available here.
3. “The Bohr Model”, University of Rochester. Available here.
Image Courtesy:
1. “Rutherford atom” By Own work (CreateJODER Xd Xd) (CC BY-SA 3.0) via Commons Wikimedia
2. “Bohr atom model English” By Brighterorange – Created by Brighterorange, based on GFDL/cc image: Bohratommodel.png. (CC BY-SA 3.0) via Commons Wikimedia

