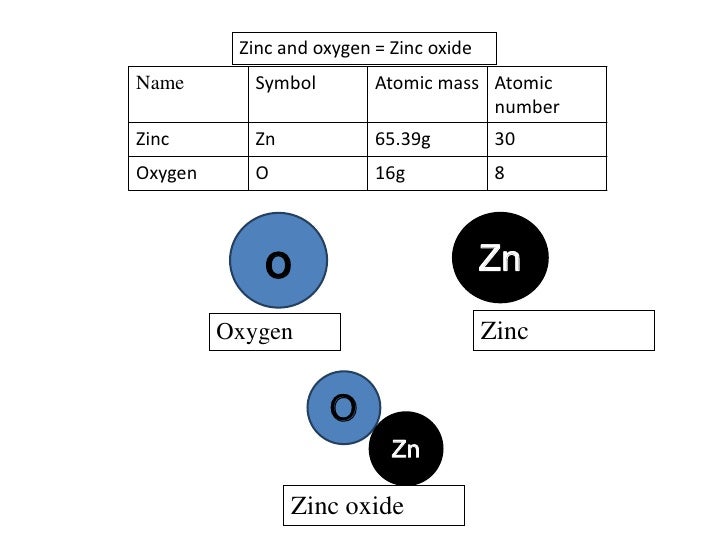
Atomic Number of Zinc is 30.
Chemical symbol for Zinc is Zn. Number of protons in Zinc is 30. Atomic weight of Zinc is 65.38 u or g/mol. Melting point of Zinc is 419,6 °C and its the boiling point is 907 °C.
» Boiling Point» Melting Point» Abundant» State at STP» Discovery YearRepresented in the periodic table as Zn, zinc is a transition metal, grouped with cadmium and mercury. With the middling atomic number 30, it has five stable isotopes of atomic weight from the dominant zinc 64 to zinc 70, plus an extra 25 radioisotopes.
- Zinc has the element symbol Zn and atomic number 30, making it a transition metal and the first.
- Zinc is a lustrous bluish-white metal. It is found in group IIb of the periodic table.It is brittle and crystalline at ordinary temperatures, but it becomes ductile and malleable when heated between 110°C and 150°C.
About Zinc
Zinc is a softish metal of light grey color and is one of so called transition metals. Its name comes from a similar German word which is believed to be a copy of an old Persian word meaning stone. It is impossible to find pure zinc on our planet, but it can be extracted from a number of minerals and compounds. Zinc is essential for normal chemical processes in living organisms, and in micro-doses it exists in our enzymes. Large or even medium dozes of zinc can be toxic. This metal is widely used as a galvanizer, in such products as lamp posts, some parts of automobiles, etc. As a part of an alloy with copper called brass, zinc is used for producing parts of musical instruments, and zinc is also used to produce many other alloys. Oxides of this chemical element are used for producing rubber, plastic, textiles, soaps, cosmetics, electrical components, and many other goods.
Properties of Zinc Element
| Atomic Number (Z) | 30 |
|---|---|
| Atomic Symbol | Zn |
| Group | 12 |
| Period | 4 |
| Atomic Weight | 65.38 u |
| Density | 7.134 g/cm3 |
| Melting Point (K) | 692.88 K |
| Melting Point (℃) | 419,6 °C |
| Boiling Point (K) | 1180 K |
| Boiling Point (℃) | 907 °C |
| Heat Capacity | 0.388 J/g · K |
| Abundance | 70 mg/kg |
| State at STP | Solid |
| Occurrence | Primordial |
| Description | Transition metal |
| Electronegativity (Pauling) χ | 1.65 |
| Ionization Energy (eV) | 9.3942 |
| Atomic Radius | 135pm |
| Covalent Radius | 131pm |
| Van der Waals Radius | 139 |
| Valence Electrons | 2 |
| Year of Discovery | prehistoric |
| Discoverer | unknown |
What is the Boiling Point of Zinc?
Zinc boiling point is 907 °C. Boiling point of Zinc in Kelvin is 1180 K.
What is the Melting Point of Zinc?
Zinc melting point is 419,6 °C. Melting point of Zinc in Kelvin is 692.88 K.
Zn Atomic Number
How Abundant is Zinc?
Abundant value of Zinc is 70 mg/kg.
What is the State of Zinc at Standard Temperature and Pressure (STP)?
Zinc Element
State of Zinc is Solid at standard temperature and pressure at 0℃ and one atmosphere pressure.
When was Zinc Discovered?
Zinc was discovered in prehistoric.
What is the electron configuration for Zn2+?
:max_bytes(150000):strip_icc()/zinc-56a12a7c5f9b58b7d0bcac9e.jpg)
1 Answer
The electron configuration of
Explanation:
The atomic number of zinc is 30, which means that all zinc atoms have 30 protons in their nuclei. A neutral atom has equal numbers of protons and electrons, so a neutral atom of zinc would have 30 electrons.
The electron configuration of a neutral zinc atom is
Zn Atomic Mass Number
The
Zinc is a d-block element, also known as a transition element. For the d-block elements, the outermost s-sublevel has higher energy than the d-sublevel, which is contrary to what the Aufbau diagram indicates.
When d-block elements lose electrons, they lose the highest energy s electrons first, which in the case of zinc are the two 4s electrons. Having eight 3d electrons and two 4s electrons is much less energetically stable than ten 3d electrons and no 4s electrons.
http://chemguide.co.uk/atoms/properties/ionstruct.html
http://chemguide.co.uk/atoms/properties/3d4sproblem.html#top
Zn-66 (atomic Number 30) Has
Related questions
